By any clinical measure, iodinated contrast is a workhorse of modern cardiology. It makes coronary arteries visible during angiography, guides stents into place, and helps physicians act fast. But it’s also transient, washing out in seconds, and it can damage the kidneys, especially in patients who already have renal disease. For Dr. Jen Streeter, an invasive cardiologist at the University of Iowa, that tradeoff was no longer acceptable.
Her response is bold and two-pronged: build a campus-wide pipeline of living, patient-derived human heart tissue for research, and use that platform to invent a safer, more durable way to see the coronary arteries during procedures. Together, the Iowa Living Human Heart Research Program (ILHHRP) and Radiopaque Aptamer Dimers (RADs) show how clinician-led insight, rigorous science, and cross-campus support can accelerate ideas with real patient impact.
A new kind of contrast: RADs
Streeter’s clinical problem statement is uncomplicated and urgent: today’s contrast agents don’t last long enough and can injure the kidneys. RADs aim to change both.
The concept: engineer RADs: small, structured nucleic acid molecules (aptamers) that selectively bind to targets in human coronary arteries and carry a radiopaque tag visible under x-ray. Instead of passively filling the vessel and vanishing, RADs would attach to the vessel wall and remain visible long enough to guide decisions with fewer reinjections and a lower total contrast dose.
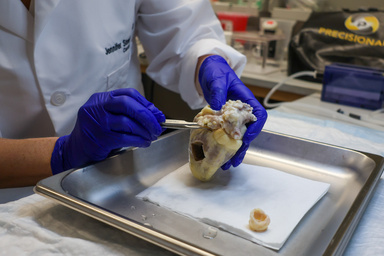
The technical lift is substantial. Aptamer “SELEX” selection typically happens in cell culture, which lacks the flow, lipids, glucose, and hormonal signals that shape real endothelial behavior. Streeter’s insight was to run selections directly on living human coronary arteries, enriching candidates that bind where it matters most: in human tissue. Starting with extraordinarily large aptamer libraries (with a quadrillion unique sequences), her team performs multi-round selections across multiple human hearts, iteratively narrowing to a small slate of candidates. The top performers will be conjugated to radiopaque tags, validated on the bench, and then tested in a pig model, a close physiological match for x-ray imaging and interventional workflows.
The Iowa Living Human Heart Institute: building a living bridge to human biology
RADs are possible because Streeter first tackled a larger bottleneck: access to viable adult human heart tissue. Through the Iowa Living Human Heart Research Program (ILHHRP), she established a pathway by working with surgical teams and pathology to enable recovery of explanted hearts from transplants and donor hearts consented for research using transplant-grade perfusion buffers and cold storage. The result: up to eight days of tissue viability for experiments that simply can’t be done on fixed or frozen samples.
What makes Iowa stand out is scale and access. While a handful of labs worldwide can perform cardiac “slice” work, Iowa now uses all parts of the heart and distributes living tissue to a broad campus community. In the past year, supply has grown from ~1 heart/month (transplant sources) up to ~1 heart/week (with support from the Iowa Donor Network). To date, the program has served about 19 labs, enabling everything from cell-signaling measurements in adult human heart sections to gene-expression interventions; capabilities long considered out of reach in standard cardiovascular research.
Crucially, ILHHRP currently distributes tissue pro bono to remove cost barriers (market value $3,000–$5,000 per specimen). In return, investigators cite and budget for the resource in their grants and manuscripts. In the last six months, 5–7 grant applications have included support for the program, and at least three manuscripts drawing on its tissue are slated for submission this year.

The enterprise has also become a training ground: two post-baccalaureate research interns and multiple undergraduates learn OR collection, lab dissection, and distribution logistics, while a specialized research technician role is being explored to sustain growth.
Where innovation meets infrastructure: UI Ventures and the Office of Innovation
None of this happens in a vacuum. Streeter’s path to RADs and ILHHRP has been closely supported by UI Ventures and the Office of Innovation—from early exposure to mentors and translational know-how, to funding strategy and credibility-building.
Catalyzing moments: Streeter connected with UI Ventures through a JPEC pitch competition and a UI Ventures funding seminar, meeting translational champions and external entrepreneurs. Those conversations led to regular check-ins that provided accountability and “next-step nudges” amid the demands of clinical care and teaching.
De-risking & funding: UI Ventures encouraged and guided the successful GAP Fund application ($20,000), creating a structured workplan and milestones that supported RAD Conjugation Optimization (RADCO) This support led to additional funding from the Carver Trust Fund.
Credibility & connections: For Streeter’s Moore Foundation application, UI Ventures coordinated letters of support from respected leaders, reinforcing the scientific case and the project’s translational potential, and aligned the proposal with campus strengths and external expectations.
This is the translational flywheel that the Office of Innovation exists to power: clinicians and scientists spot real-world problems; programs like ILHHRP provide differentiating infrastructure; and UI Ventures converts early promise into a plan, funding, partners, evidence, and timelines that can carry an idea toward impact.
Additional information:
