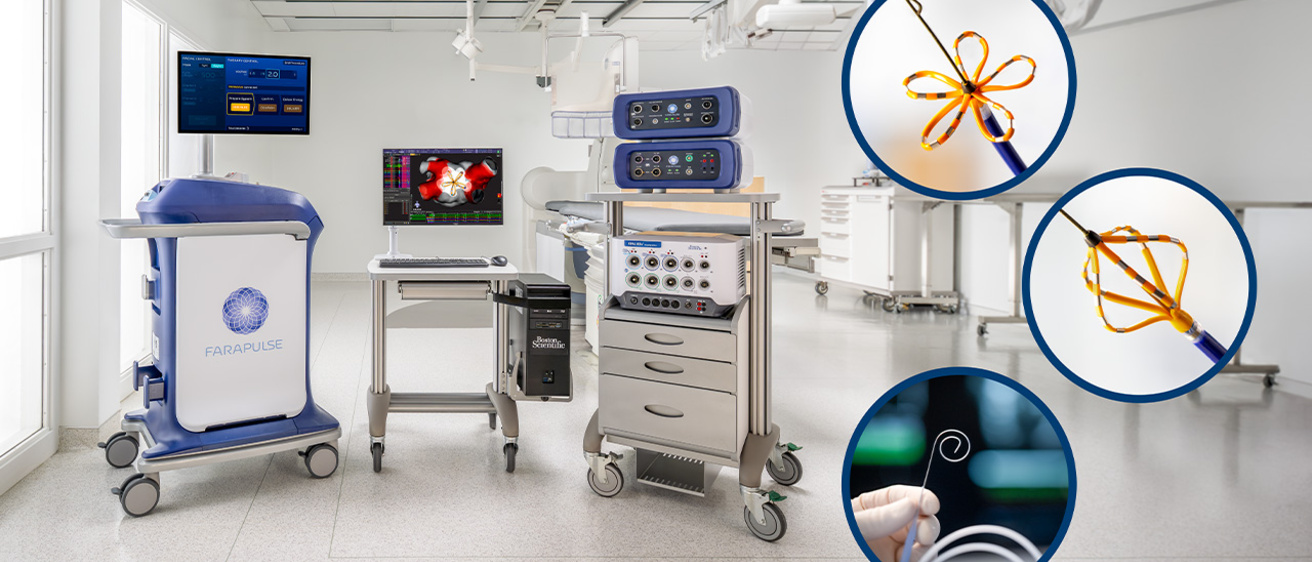
Boston Scientific Corporation (NYSE: BSX) has received U.S. Food and Drug Administration (FDA) approval to expand the instructions for use (IFU) labeling for the FARAPULSE™ Pulsed Field Ablation (PFA) System. The updated labeling now includes approval for the system in the treatment of drug refractory, symptomatic persistent atrial fibrillation (AF), an arrythmia in which the heart beats abnormally for at least seven days.
AF affects an estimated 59 million people worldwide and many have the persistent form of the condition, which can cause dizziness, fatigue, shortness of breath and increase the risk of stroke. The FARAPULSE PFA System treats AF by delivering pulsed field energy through a catheter to ablate heart tissue. This approval updates the IFU for both the FARAWAVE™ PFA Catheter and the FARAWAVE NAV™ PFA Catheter to include treatment for patients with persistent AF.
"Backed by clinical evidence and our global commercial experience, this update advances our efforts to further shape the future of AF treatment with safe and effective ablation technologies," said Brad Sutton, M.D., chief medical officer, AF Solutions, Boston Scientific. "We look forward to studying the system in new clinical trials, including patients in need of re-do ablations and those with more complex arrhythmias, which account for a large portion of the procedures today still using thermal ablation."
The FDA approval for expanded labeling was supported by clinical evidence from phase one of the ADVANTAGE AF clinical trial presented at AF Symposium 2025 and recently published in the Journal of the American College of Cardiology and met both the primary safety and effectiveness endpoints. In the prospective, single-arm trial, 260 patients who were drug intolerant to at least one Class I/III anti-arrhythmic drug (AAD) were enrolled at 43 global sites. There were no reported incidences of stroke, pulmonary vein stenosis, atrio-esophageal fistula or major access complications and the symptomatic AF recurrence-free rate was 85.3%. Observationally, among physicians that performed three or more procedures, the symptomatic recurrence-free rate increased to 91.4%.
Boston Scientific anticipates CE mark as well as approval in Japan and China in the coming months. The company also recently initiated the ReMATCH IDE clinical trial, which will study approximately 375 patients across 40 centers in the U.S. and Asia. The study will evaluate the safety and effectiveness of the FARAWAVE PFA Catheter for posterior wall ablation and pulmonary vein isolation in patients with persistent AF who previously received an ablation with a PFA, radiofrequency or cryoablation catheter and experienced a recurrence of the condition. It will also evaluate adjunctive use of the FARAPOINT™ PFA Catheter* for cavotricuspid isthmus ablation and left atrial ablation of the mitral isthmus in the same patient population.
More information on the FARAPULSE PFA System is available here.